Videos & Webinars
Hormonal Contraception and HIV Risk: Understanding the ECHO Trial
Previous

FP2020 and Jhpiego Promote the Importance of Postp...
Next

My Age Zimbabwe: Youth Accountability on FP2020 in...
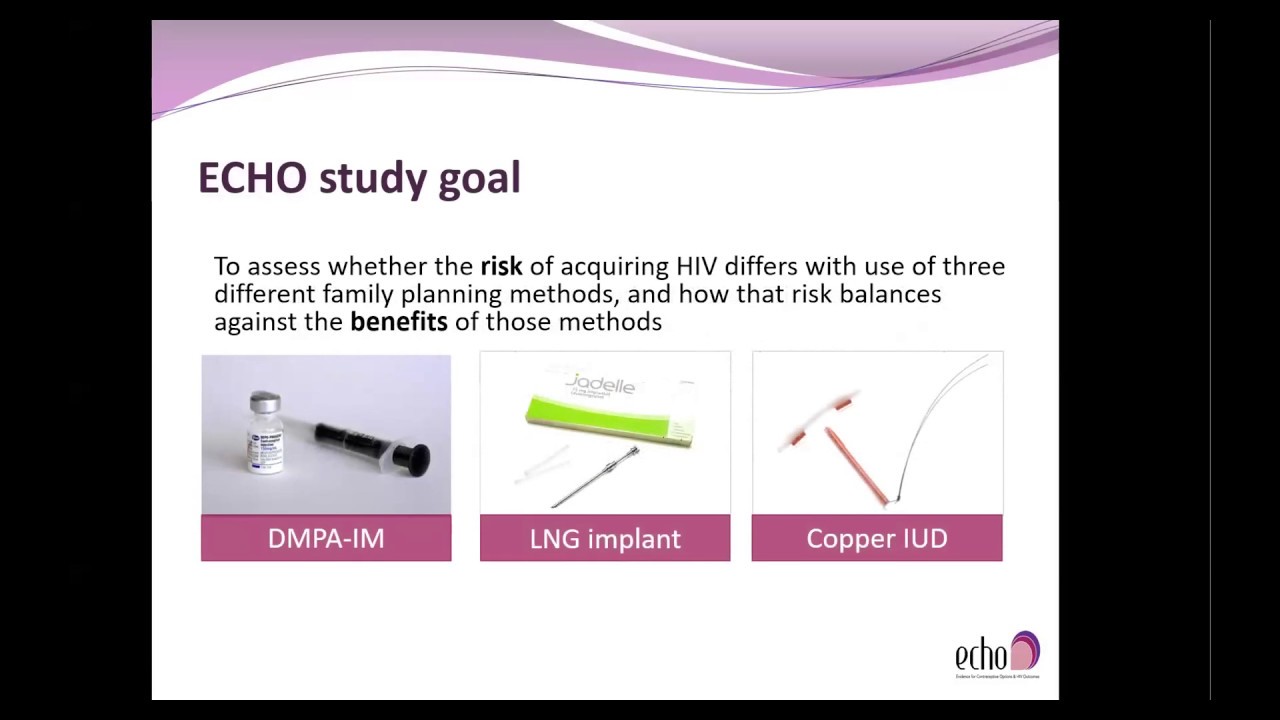
The Evidence for Contraceptive Options and HIV Outcomes (ECHO) Study is an open-label, randomized, clinical trial comparing three highly effective, reversible methods of contraception — the progestogen-only injectable depot-medroxyprogesterone acetate (DMPA), a levonorgestrel implant, and the non-hormonal copper intrauterine device — to evaluate whether there is any difference in the risk of acquiring HIV infection among users of these methods. Results, expected in mid-2019, will help guide the implementation of safe, effective policies and services that will enable women at high risk of HIV to make fully informed choices about contraception and HIV prevention.